Resources
Analytical Solutions for Residual Nucleic Acid Detection in Gene Therapy Vectors
Vector Quality Control: Residual Host Cell DNA
ProtaGene Authors: Matteo Franco, Sandra Pacharra, PhD, Nicolas Brauckhoff, PhD, Irene Gil-Farina, PhD
More than 25 years after the first clinical AAV study, advances in vector design and delivery allowed for an increased therapeutic efficacy turning recombinant AAVs into the platform of choice for in vivo gene therapy. At the same time, the improvements in all stages of vector manufacturing have played an essential role in this evolution and will be decisive to place AAVs as an accessible therapeutic option.
“Our team of experts have a strong track record providing in-depth solutions tailored to your specific product or to support regulatory packages.“
With rAAV vectors being directly administered to the patient and manufacturing processes still under development, there is a need for concomitantly evolving bioanalytical assays to guarantee the safety and efficacy of the drug product. The detection and quantification of residual DNA is an essential part of this safety assessment and unanimously required by regulatory agencies. ProtaGene’s analytical platform provides complementary solutions that our customers can leverage to support their manufacturing process or product development and optimization, as well as to later ensure that every vector batch meets the release quality specifications.
Next-Generation Sequencing: Unbiased and Multiplexed Detection
The combination of next generation sequencing (NGS) and dedicated data analyses provides a versatile and powerful tool for the identification and quantification of DNA species present within vector batches. These studies rely on the ligation of adaptors allowing for sequencing to every nucleic acid molecule and the subsequent data analysis by comparing the sequencing reads retrieved to any reference sequence of interest. Therefore, this approach simultaneous detects impurities arising from multiple sources (helper plasmids, host cell DNA, etc) without requiring the presence of specific primer binding sites but still allowing to interrogate whether specific regions of those species are present (for example antibiotic resistance or potentially oncogenic genes). The vector genomes are also represented in this dataset thus allowing for additional assessments such as product identity or integrity monitoring. Besides, our expert team has a strong track record providing in-depth solutions tailored to your specific product or to support regulatory packages.
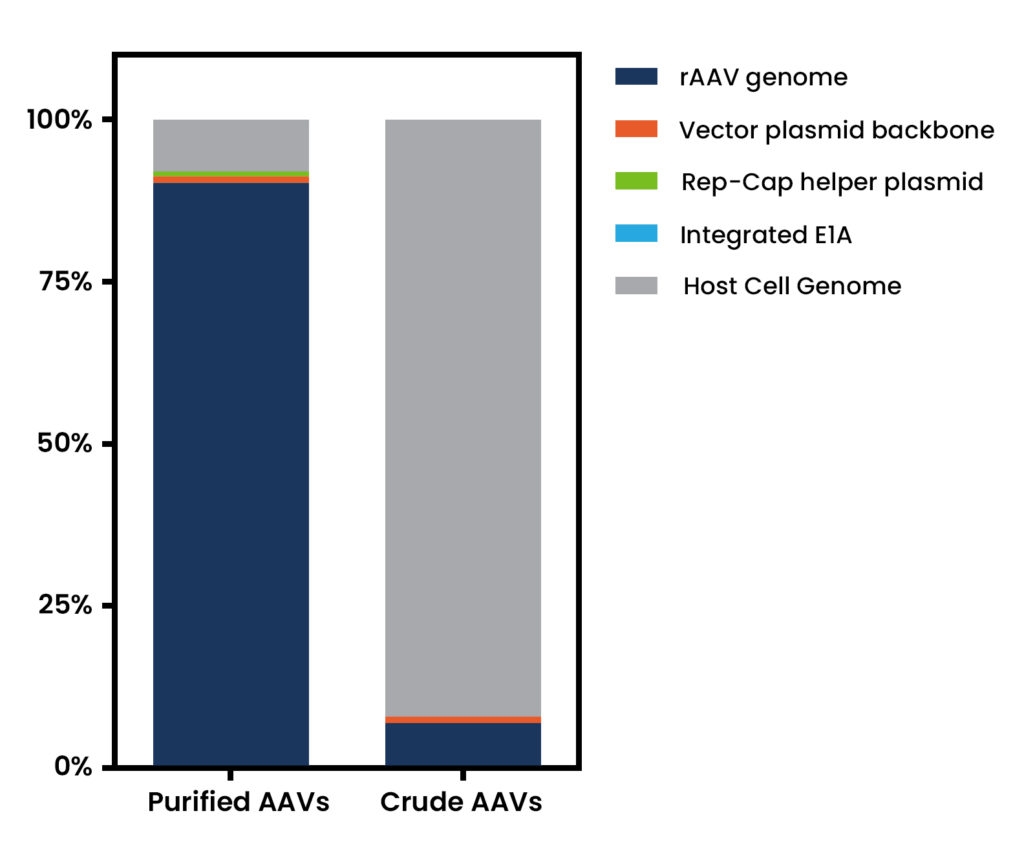
Figure 1. NGS analysis of DNA species present in purified and crude rAAV vector batches. Analyses considered nucleic acid sequences arising from: the rAAV vector genome, the vector plasmid backbone, the Rep-Cap helper plasmid, the host cell genome and, specifically, the E1A region integrated within the host cell genome.
Quantitative PCR: Rapid and Sensitive Testing
The quantitative PCR (qPCR) is still the gold standard approach for a fast and robust detection of known nucleic acid species. These assays enable high specificity and sensitivity in the absolute quantification of DNA impurities within your vector batches. ProtaGene offers universal assays for the quantification of residual host cell DNA in vector preparations. In addition, we put at your service our broad experience in qPCR development to generate assays specifically designed for your therapeutic vector or manufacturing platform providing a highly reliable residual DNA detection.
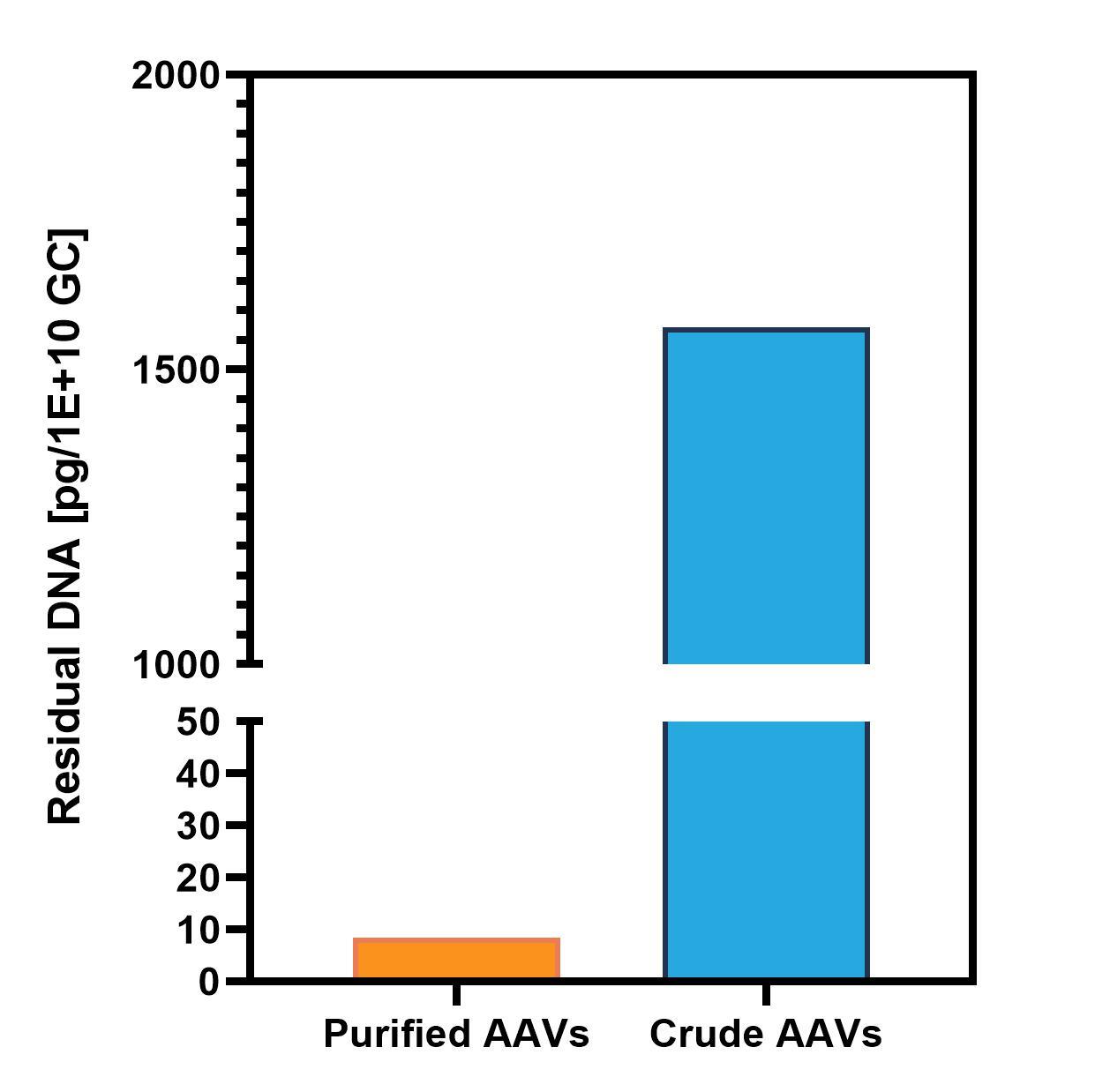
Figure 2. Absolute quantification of host cell DNA in purified and unpurified rAAV batches using qPCR.
Customized and Synergistic Solutions
Considering the two complementary resolution levels of NGS and qPCR, our nucleic acid impurity detection portfolio provides a unique opportunity to design a personalized testing scheme to support your gene therapy programs at each stage of the drug development process. Our experience and company compromise with quality and compliance guarantee the appropriate scenario to establish your gene therapy vector testing site.
Subscribe to Our Email List
Talk to Our Experts
Whether you are looking to learn more about our platforms or get a project started, contact our team today to discover how ProtaGene can support your development programs.
Contact Us