Molecular Bioanalysis
Molecular Biology Tools to Unlock the Full Potential of Your Gene Therapy Products
Biodistribution, transgene expression, and vector shedding are critical components of a gene therapy molecule’s development, providing crucial data for IND-enabling preclinical studies and safety evaluation during clinical development.
Gene therapy carries potential risks, including unintended effects caused by the gene therapy vector. Biodistribution studies are critical for determining the distribution and persistence of the gene therapy vector in target and non-target tissues and are generally combined with transgene expression assessments to aid interpreting any observed adverse effects from the toxicology studies.
A key aspect of clinical safety involves understanding the potential for vector shedding of the administered gene therapy. Evaluation of biofluids informs understanding of personal and environmental risks and is also a recommended part of preclinical development. Inadequate safety data could lead to regulatory approval challenges and delay time to the clinic.

Speed Time to Market with Cutting-Edge Molecular Bioanalysis Approaches
Our molecular bioanalytical solutions are designed to enable preclinical assessment of safety risks and monitoring of gene therapies in the clinic.
ProtaGene offers:
- Expert understanding of industry expectations for developing and validating molecular assays
- Established processes that maintain the integrity of sample analysis results while minimizing contamination risks
- State-of-the-art instrumentation and automation to enable high-throughput analysis of tissues, blood, and biofluids
- Bioanalytical labs designed and managed by a leading industry expert with 25+ years of experience
- New modern facilities, including automation, run under GLP compliance
Molecular Bioanalytical Solutions
ProtaGene offers comprehensive bioanalytical solutions critical for analyzing viral vector performance and safety.
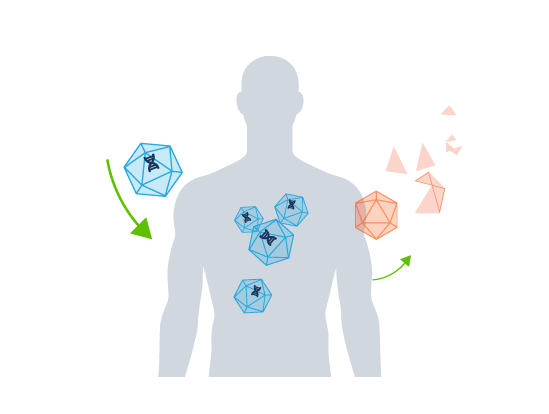
Biodistribution & Transgene Expression
Gene therapies (for example, adeno-associated viral (AAV) and adenoviral (AdV) vectors) are targeted therapies administered to patients to treat a range of monogenic diseases. Safety and efficacy rely on the delivery of the therapeutic to the target tissue.
Uptake by non-target tissues is common, risking patient safety and therapeutic effectiveness. Biodistribution studies are conducted during preclinical development to evaluate how gene therapies are taken up and retained in vivo. Understanding the biodistribution of a product in preclinical models is essential for assessing the risk during preclinical gene therapy development.
-
The levels delivered to diverse tissues and the persistence of the gene therapy vector are monitored over time to confirm the uptake and retention of the viral vectors in target and non-target tissues.
-
Expression levels of the therapeutic transgene are also assessed in tissues shown to contain the vector.
-
Biodistribution results are combined with the transgene expression levels to enable an understanding of the correlations between dose, biodistribution, and toxicology of the gene therapy vectors.
Vector Shedding
Viral vectors used for in vivo gene delivery (e.g., AAV) can be shed from the patient via biofluids following administration, and persistent levels can indicate that unwanted replication of the vector is occurring.
Therefore, vector shedding data provides critical information during the clinical development of these types of products.
-
Vector shedding analysis detects and quantifies the vector DNA in biofluids from dosed animals or patients, such as blood, saliva, tears, urine, semen, and feces.
-
Understanding vector shedding is expected during preclinical development to provide early indications of replication potential.
