Resources
Host Cell Protein Detection and Analysis—4 Things You Need to Know
Host cell proteins (HCPs) are impurities present in all therapeutics derived from biological sources used for manufacturing and comprise a significant portion of process-related impurities within biologic products. Due to product safety and efficacy risks, a drug product’s overall quantity of residual HCPs as well as the presence of high-risk HCP is a critical quality attribute (CQA) that must be characterized and controlled throughout the manufacturing process.1
Regulatory requirements specify the need for robust analytical methods that monitor the removal of HCPs in both process development and commercial manufacturing scenarios. While HCP analysis and clearance is a complex endeavor, there are four foundational issues discussed in this article—HCP risks and detection challenges, ELISA’s role and shortcomings in HCP detection, the use of mass spectrometry (MS) as a highly effective identification tool and orthogonal method, and the risk reduction offered by the combination of ELISA and MS.

1. HCP Risks & Detection Challenges
HCPs are viewed as a CQA in biotechnology products because they present potential safety risks for patients and can reduce drug efficacy, stability, and product quality.1 Specifically, because they are proteins foreign to the human body, they can elicit potentially dangerous toxicity or immune responses; in other cases, they can negatively impact drug product stability and efficacy.
Therefore, HCPs must be analyzed for product release, and to control risk at release, products are characterized more deeply using robust detection methods and quantification throughout the downstream processes (DSP) and in the final drug substance (DS). Developing an understanding of the correspondence between upstream processes (USP) and DSP with the HCP profile of the DS is valuable for mitigating risk in product development.
The diversity of cell lines producing today’s biologics continues to expand as innovations drive the ability to increase production yields and better control desirable attributes. However, the multitude of disparate cell lines, from mammalian, insect, and plant cells to prokaryotic cells (such as E. coli), includes tens of thousands of genes, producing widely varying HCPs with diverse physicochemical and immunological properties. It is challenging to develop and maintain effective detection and characterization protocols that effectively address the diverse spectrum of potential impurities.
HCP detection challenges include the need to detect low levels of residual HCPs in the presence of a huge excess of the biologic drug and the reality that the population of HCPs can change during process development. Ultimately, to demonstrate robust bioprocesses, appropriate methods for analyzing HCPs are needed to support process development, process validation, and control system testing.
2. ELISA’s HCP Detection Role & Shortcomings
Immunoassay, most commonly in the form of sandwich enzyme-linked immunosorbent assay (ELISA), has been and continues to be regarded as the gold-standard method for monitoring HCP clearance. ELISA’s high sensitivity, high throughput, and established position in the market allowed it to earn the reputation as the “workhouse” of HCP detection.
However, HCP determination by ELISA does have a key shortcoming that poses a potential safety risk. Arguably, ELISA’s most significant limitation in HCP detection and monitoring is that these assays rely on custom immunoreagents, which are generated using crude protein material from the host cell to raise polyclonal antibody reagents (pAb) in different animal species such as rabbit, goat or sheep. As such, the pAb immunoreagent is comprised of an undefined, highly diverse mixture of antibodies to a complex set of protein targets, and as a result, the assay is limited by the level and quality of coverage of HCPs by the pAbs used for detection.
pAbs are raised against a highly diverse HCP population, often an USP sample, in which some species may be overrepresented. Additionally, immunoreagents generated using a DSP sample can be lacking in HCPs that then may appear in a downstream purification process due to variability or as the result of a DSP change. Because not all HCPs are equally represented in any one sample and individual HCPs have varying degrees of immunogenicity, the use of immunoreagents leads to gaps in HCP detection by ELISA methods.
Process-specific HCP ELISA is also expensive and time-consuming, given the need for process-specific reagents, antibodies, assay development, and validation.
3. Advanced Mass Spectrometry is a Highly Effective Tool for Evaluating HCPs and Related Risks
Mass spectrometry has proven to be an extremely effective method for identifying and quantifying process-related HCP impurities using a proteomics-based approach (shotgun MS/MS). The analysis provides identification of the individual HCP species present in a sample down to equally low levels of sensitivity as ELISA methods. Notably, mass spectrometry is a direct analysis method and does not require custom immunoreagents, providing a consistent assessment tool to evaluate HCP content.
Knowing the composition of HCPs in process development samples enables a better understanding of potential risks and enables informed decision-making (Figure). There are many examples of recent proteomics studies that have demonstrated sensitive detection for identifying low-abundance HCPs in purified Drug Substance (DS). There are many examples of recent proteomics studies that have demonstrated sensitive detection for identifying low-abundance HCPs in purified DS. Additionally, MS is used to characterize upstream and downstream process influences on resulting HCP patterns for different recombinant protein products.2
MS technology is of tremendous advantage in that it can detect individual HCPs, which is needed for a thorough understanding of the purification process and is particularly helpful for process optimization and mitigating risk.
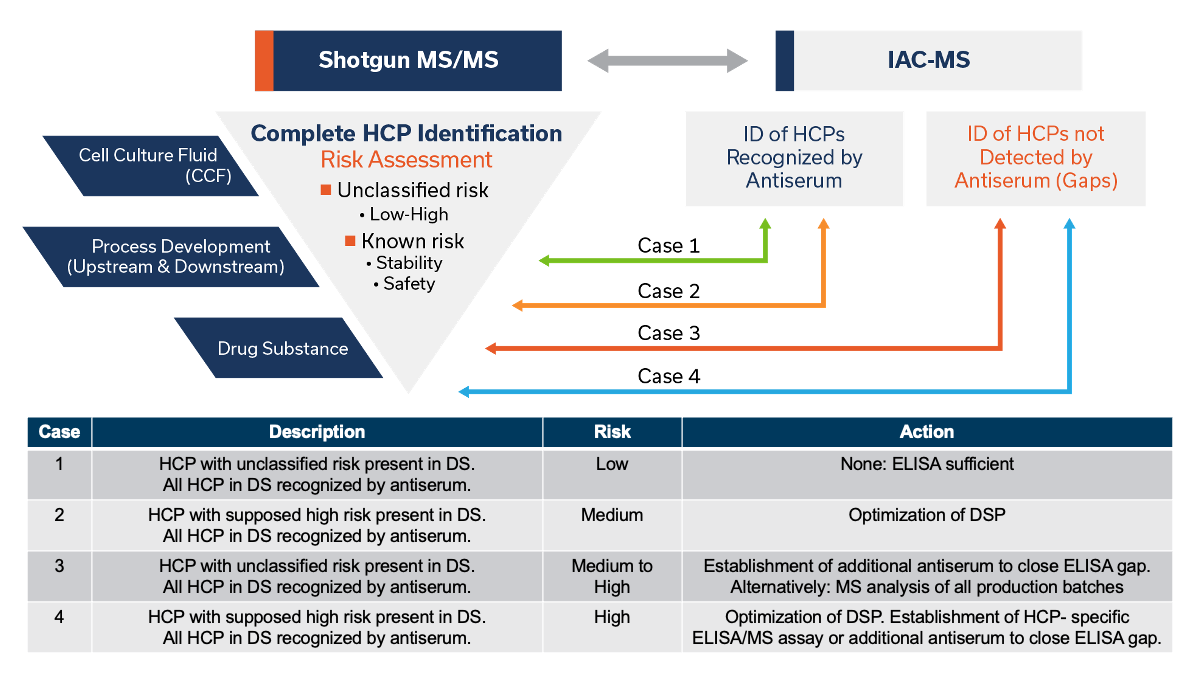
4. Risk Mitigation by Evaluation of ELISA Immunoreagent Coverage by IAC-MS
ELISA remains the primary, nearly universal method for monitoring HCPs in biologic products at release because of its ease of use in a GMP environment. MS adds significant value by providing complementary information about the HCP content in a product. This advantage also has been leveraged with great success to evaluate coverage of HCPs by the pAbs in ELISA immunoreagents.
The immunoaffinity chromatography-MS (IAC-MS) approach enables understanding of the immunoreagent’s ability to detect HCPs (Figure), and this information has been used to evaluate potential safety and stability risk (Table) and to guide ELISA kit development to achieve consistent and more complete detection of HCP content in DS by ELISAs. IAC-MS involves analyses of the pAbs’ ability to capture HCPs and is used to identify which HCPs are missed by the immunoreagent (gaps). By correlation of the HCP present in the DS and observed ELISA detection gaps respective action for risk mitigation can be performed (Table).
In short, MS is a valuable orthogonal technique to assure product quality and increase safety for the patient. To learn more about ProtaGene’s HCP MS analysis capabilities, view our application note—Mass Spectrometry-based Host Cell Protein Analysis in Biologics Development.
1. Host-Cell Protein Measurement and Control, BioPharm, May 2015
2. Host cell protein detection gap risk mitigation: quantitative IAC-MS for ELISA antibody reagent coverage determination, MAbs, August 2021
Subscribe to Our Email List
Talk to Our Experts
Whether you are looking to learn more about our platforms or get a project started, contact our team today to discover how ProtaGene can support your development programs.
Contact Us